Periodic table puzzle answer key: a powerful tool that unlocks the secrets of chemistry, empowering puzzle solvers to conquer the enigmatic world of elements. Delve into the fascinating realm of periodic table puzzles, where strategy and knowledge intertwine to reveal the hidden order of the universe.
The periodic table, a masterpiece of scientific organization, arranges elements based on their atomic properties, creating a treasure map for chemists. With an answer key in hand, puzzle enthusiasts can navigate this map with ease, discovering the unique characteristics of each element and unraveling the intricate relationships that govern their behavior.
Periodic Table Puzzle Answer Key
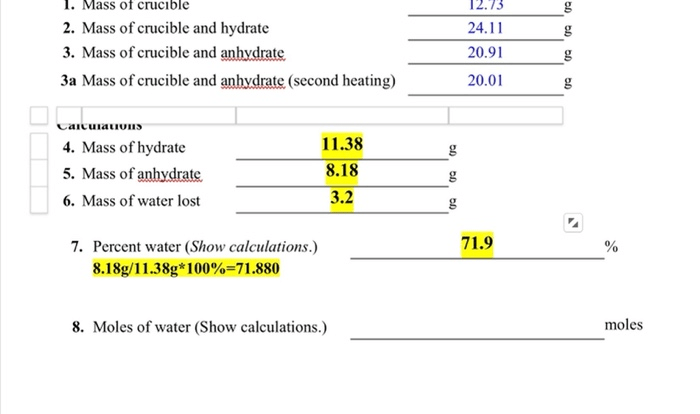
A periodic table puzzle answer key provides solutions to puzzles that involve arranging the elements of the periodic table in a specific pattern or sequence. These puzzles can vary in difficulty, from simple crosswords to complex logic puzzles.
There are numerous benefits to using an answer key for periodic table puzzles. It can help to:
Confirm Solutions
- Verify the accuracy of your puzzle solutions.
- Identify errors or misconceptions in your understanding of the periodic table.
Enhance Learning
- Provide additional insights into the organization and properties of the elements.
- Reinforce your knowledge of periodic trends and relationships.
Improve Puzzle-Solving Skills
- Develop logical reasoning and problem-solving abilities.
- Enhance your ability to analyze and interpret patterns.
Elements and Their Properties
The periodic table is a tabular arrangement of the chemical elements, organized on the basis of their atomic number, electron configuration, and recurring chemical properties. It is generally accepted that the modern periodic table was first published by Dmitri Mendeleev in 1869, although several other scientists had developed similar tables prior to this.
The periodic table is a powerful tool for organizing and understanding the chemical elements. It can be used to predict the properties of an element based on its position in the table, and it can also be used to explain the chemical reactions that occur between elements.
Elements in the Periodic Table
The periodic table contains 118 elements, which are arranged in 18 vertical columns, called groups, and 7 horizontal rows, called periods. The elements are arranged in order of increasing atomic number, which is the number of protons in the nucleus of an atom.
| Group | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Period 1 | H | He | ||||||||||||||||
| Period 2 | Li | Be | B | C | N | O | F | Ne | ||||||||||
| Period 3 | Na | Mg | Al | Si | P | S | Cl | Ar | ||||||||||
| Period 4 | K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr |
| Period 5 | Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe |
| Period 6 | Cs | Ba | * | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn |
| Period 7 | Fr | Ra | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og |
*Lanthanides: La, Ce, Pr, Nd, Pm, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb, Lu
Actinides: Th, Pa, U, Np, Pu, Am, Cm, Bk, Cf, Es, Fm, Md, No, Lr
Organization of the Periodic Table
The periodic table is organized based on the element’s atomic number, electron configuration, and recurring chemical properties. The elements are arranged in order of increasing atomic number, which is the number of protons in the nucleus of an atom.
The periodic table is divided into four blocks: the s-block, the p-block, the d-block, and the f-block. The s-block and p-block elements are located in the main groups of the periodic table, while the d-block and f-block elements are located in the transition metals and inner transition metals, respectively.
Trends in Element Properties, Periodic table puzzle answer key
There are a number of trends in element properties that can be observed across the periodic table. These trends include:
- Atomic radius: The atomic radius generally increases down a group and decreases across a period.
- Ionization energy: The ionization energy generally increases across a period and decreases down a group.
- Electronegativity: The electronegativity generally increases across a period and decreases down a group.
- Metallic character: The metallic character generally increases down a group and decreases across a period.
- Nonmetallic character: The nonmetallic character generally increases across a period and decreases down a group.
These trends can be explained by the changes in the number of electrons and the nuclear charge of the elements across the periodic table.
Puzzle Solving Strategies
Solving periodic table puzzles can be an enjoyable and rewarding challenge. By following a few simple strategies, you can increase your chances of success.
One of the most important strategies is to familiarize yourself with the periodic table. This will help you to identify the elements and their properties quickly and easily. Another helpful strategy is to start with the easier puzzles and work your way up to the more difficult ones.
This will help you to build your confidence and learn from your mistakes.
Using the Answer Key
The answer key can be a valuable tool for checking your solutions. However, it is important to use it wisely. If you are stuck on a puzzle, do not immediately turn to the answer key. Instead, try to solve the puzzle on your own first.
This will help you to learn and improve your problem-solving skills.
Once you have solved the puzzle, you can use the answer key to check your solution. If your solution is incorrect, compare it to the correct solution in the answer key. This will help you to identify your mistakes and learn from them.
Example
Here is an example of a solved puzzle:
Across 1. A noble gas (6 letters) HELIUM 2. The element with the atomic number 1 (1 letter) H 3. A metal used in making coins (3 letters) CU 4. A nonmetal that is essential for life (2 letters) O 5. A metalloid that is used in semiconductors (4 letters) SI
Down 1. A halogen that is used in bleach (2 letters) CL 2. A transition metal that is used in steel (3 letters) FE 3. A lanthanide that is used in magnets (3 letters) ND 4. An actinide that is used in nuclear reactors (3 letters) U
This puzzle can be solved by using the strategies Artikeld above. First, familiarize yourself with the periodic table. Then, start with the easier clues and work your way up to the more difficult ones. Finally, use the answer key to check your solutions.
Applications of Periodic Table Puzzles: Periodic Table Puzzle Answer Key
Periodic table puzzles are not only entertaining but also educational and useful in assessing students’ understanding of chemistry.
These puzzles can be used to reinforce students’ knowledge of the periodic table, its organization, and the properties of elements.
Educational Benefits
- Improve problem-solving skills
- Enhance critical thinking
- Develop logical reasoning
- Foster teamwork and collaboration
- Make learning chemistry more engaging and enjoyable
Assessing Student Understanding
Periodic table puzzles can be used as formative assessments to identify areas where students need additional support.
By analyzing students’ performance on these puzzles, teachers can gain insights into their understanding of:
- Element symbols and names
- Atomic numbers and atomic masses
- Periodic trends (e.g., atomic radius, electronegativity)
- Chemical properties of different elements
Games and Competitions
Periodic table puzzles are also popular in games and competitions, such as:
- Chemistry Olympiads
- Science fairs
- Online quizzes
- Board games
- Escape rooms
These puzzles add an element of fun and challenge to learning chemistry and can help students develop a deeper understanding of the subject.
Question Bank
What is the purpose of a periodic table puzzle answer key?
A periodic table puzzle answer key provides solutions to periodic table puzzles, enabling solvers to check their answers and deepen their understanding of element properties and their organization.
How can I use an answer key to solve periodic table puzzles?
Compare your puzzle solutions to the answer key to identify any errors and reinforce correct answers. The key can also guide your puzzle-solving strategies, providing insights into element relationships and patterns.
What are the benefits of using an answer key for periodic table puzzles?
Answer keys enhance the learning experience by providing immediate feedback, promoting self-assessment, and facilitating a deeper understanding of periodic table concepts.